Scientists discover how oxygen loss saps a lithium-ion battery’s voltage
Measuring the process in unprecedented detail gives them clues to how to minimize the problem and protect battery performance.
By Glennda Chui
When lithium ions flow in and out of a battery electrode during charging and discharging, a tiny bit of oxygen seeps out and the battery’s voltage – a measure of how much energy it delivers – fades an equally tiny bit. The losses mount over time, and can eventually sap the battery’s energy storage capacity by 10-15%.
Now researchers have measured this super-slow process with unprecedented detail, showing how the holes, or vacancies, left by escaping oxygen atoms change the electrode’s structure and chemistry and gradually reduce how much energy it can store.
The results contradict some of the assumptions scientists had made about this process and could suggest new ways of engineering electrodes to prevent it.
The research team from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University described their work in Nature Energy today.
“We were able to measure a very tiny degree of oxygen trickling out, ever so slowly, over hundreds of cycles,” said Peter Csernica, a Stanford PhD student who worked on the experiments with Associate Professor Will Chueh. “The fact that it’s so slow is also what made it hard to detect.”
A two-way rocking chair
Lithium-ion batteries work like a rocking chair, moving lithium ions back and forth between two electrodes that temporarily store charge. Ideally, those ions are the only things moving in and out of the billions of nanoparticles that make up each electrode. But researchers have known for some time that oxygen atoms leak out of the particles as lithium moves back and forth. The details have been hard to pin down because the signals from these leaks are too small to measure directly.
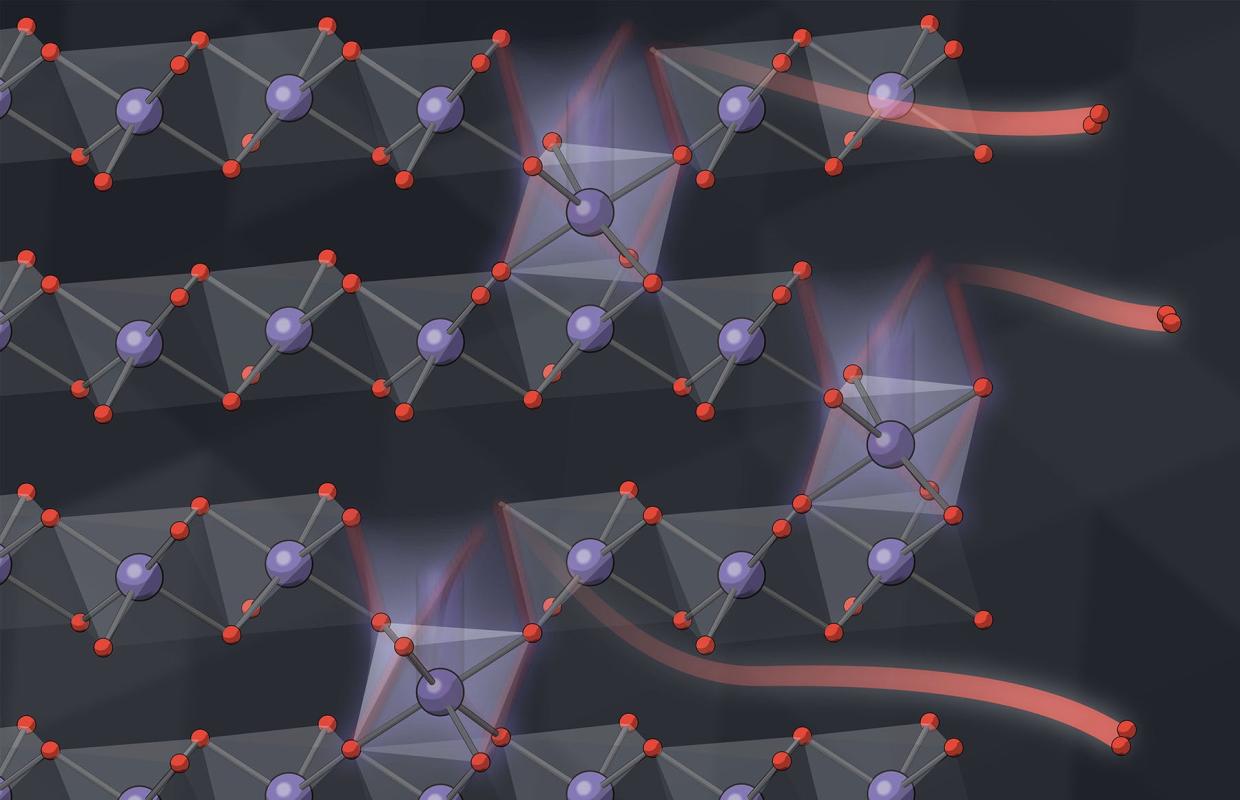
“The total amount of oxygen leakage, over 500 cycles of battery charging and discharging, is 6%,” Csernica said. “That’s not such a small number, but if you try to measure the amount of oxygen that comes out during each cycle, it’s about one one-hundredth of a percent.”
In this study, researchers measured the leakage indirectly instead, by looking at how oxygen loss modifies the chemistry and structure of the particles. They tracked the process at several length scales – from the tiniest nanoparticles to clumps of nanoparticles to the full thickness of an electrode.
Because it’s so difficult for oxygen atoms to move around in solid materials at the temperatures where batteries operate, the conventional wisdom has been that the oxygen leaks come only from the surfaces of nanoparticles, Chueh said, although this has been up for debate.
To get a closer look at what’s happening, the research team cycled batteries for different amounts of time, took them apart, and sliced the electrode nanoparticles for detailed examination at Lawrence Berkeley National Laboratory’s Advanced Light Source. There, a specialized X-ray microscope scanned across the samples, making high-res images and probing the chemical makeup of each tiny spot. This information was combined with a computational technique called ptychography to reveal nanoscale details, measured in billionths of a meter.
Meanwhile, at SLAC’s Stanford Synchrotron Light Source, the team shot X-rays through entire electrodes to confirm that what they were seeing at the nanoscale level was also true at a much larger scale.
A burst, then a trickle
Comparing the experimental results with computer models of how oxygen loss might occur, the team concluded that an initial burst of oxygen escapes from the surfaces of particles, followed by a very slow trickle from the interior. Where nanoparticles glommed together to form larger clumps, those near the center of the clump lost less oxygen than those near the surface.
Another important question, Chueh said, is how the loss of oxygen atoms affects the material they left behind. “That’s actually a big mystery,” he said. “Imagine the atoms in the nanoparticles are like close-packed spheres. If you keep taking oxygen atoms out, the whole thing could crash down and densify, because the structure likes to stay closely packed.”
Since this aspect of the electrode’s structure could not be directly imaged, the scientists again compared other types of experimental observations against computer models of various oxygen loss scenarios. The results indicated that the vacancies do persist – the material does not crash down and densify – and suggest how they contribute to the battery’s gradual decline.
“When oxygen leaves, surrounding manganese, nickel and cobalt atoms migrate. All the atoms are dancing out of their ideal positions,” Chueh said. “This rearrangement of metal ions, along with chemical changes caused by the missing oxygen, degrades the voltage and efficiency of the battery over time. People have known aspects of this phenomenon for a long time, but the mechanism was unclear.”
Now, he said, “we have this scientific, bottom-up understanding” of this important source of battery degradation, which could lead to new ways of mitigating oxygen loss and its damaging effects.
Chueh is an investigator with the Stanford Institute for Materials and Energy Sciences (SIMES) at SLAC. The Advanced Light Source, Stanford Synchrotron Radiation Lightsource and Spallation Neutron Source at Oak Ridge National Laboratory, where parts of this work were performed, are DOE Office of Science user facilities. Major funding came from the DOE Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office, and samples were provided by the Samsung Advanced Institute of Technology Global Research Outreach program.
Citation: Peter M. Csernica et al., Nature Energy, 14 June 2021 (10.1038/s41560-021-00832-7)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a vibrant multiprogram laboratory that explores how the universe works at the biggest, smallest and fastest scales and invents powerful tools used by scientists around the globe. With research spanning particle physics, astrophysics and cosmology, materials, chemistry, bio- and energy sciences and scientific computing, we help solve real-world problems and advance the interests of the nation.
SLAC is operated by Stanford University for the U.S. Department of Energy’s Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time.